CaCO3 (Calcium Carbonate)
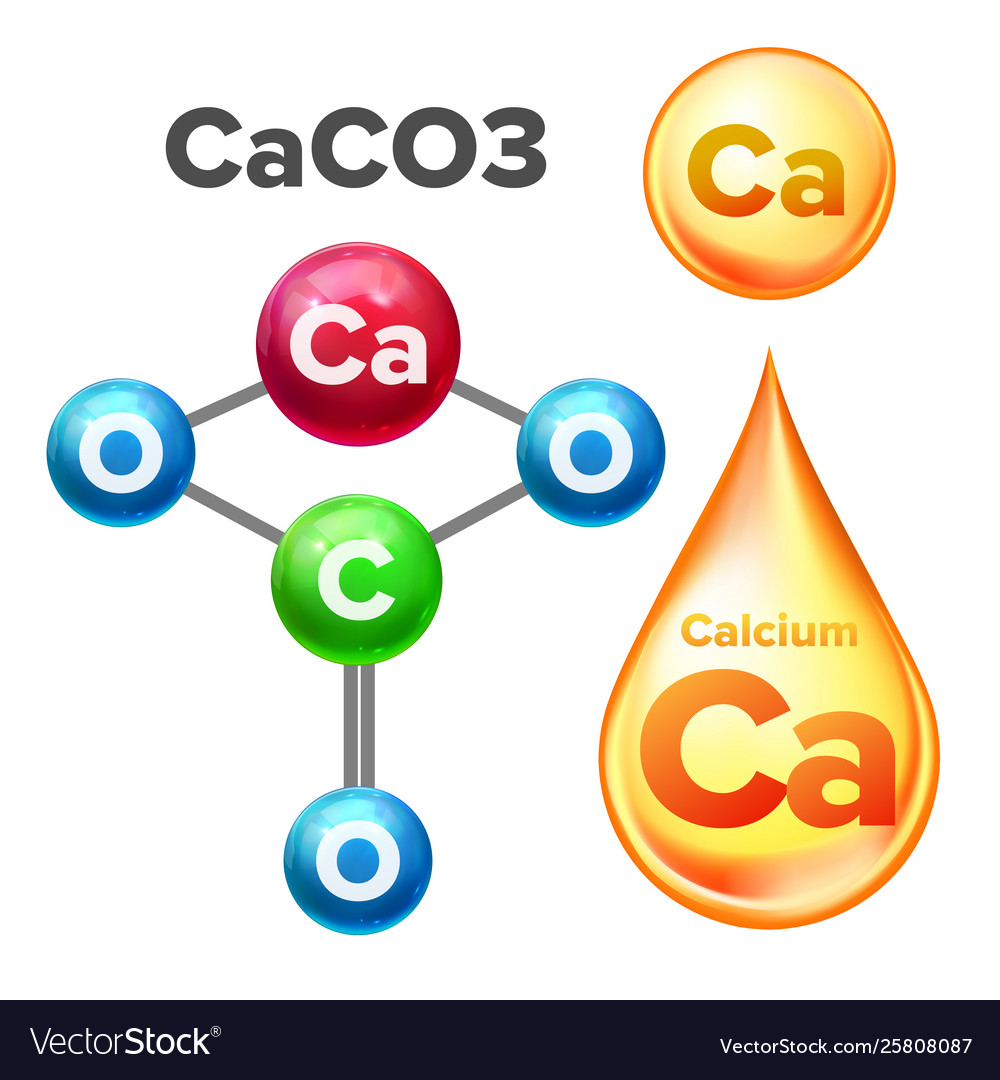
Molecular structure calcium carbonate caco3 Vector Image
The most important calcium compound is calcium carbonate, CaCO 3, the major constituent of limestone, marble, chalk, oyster shells, and corals.
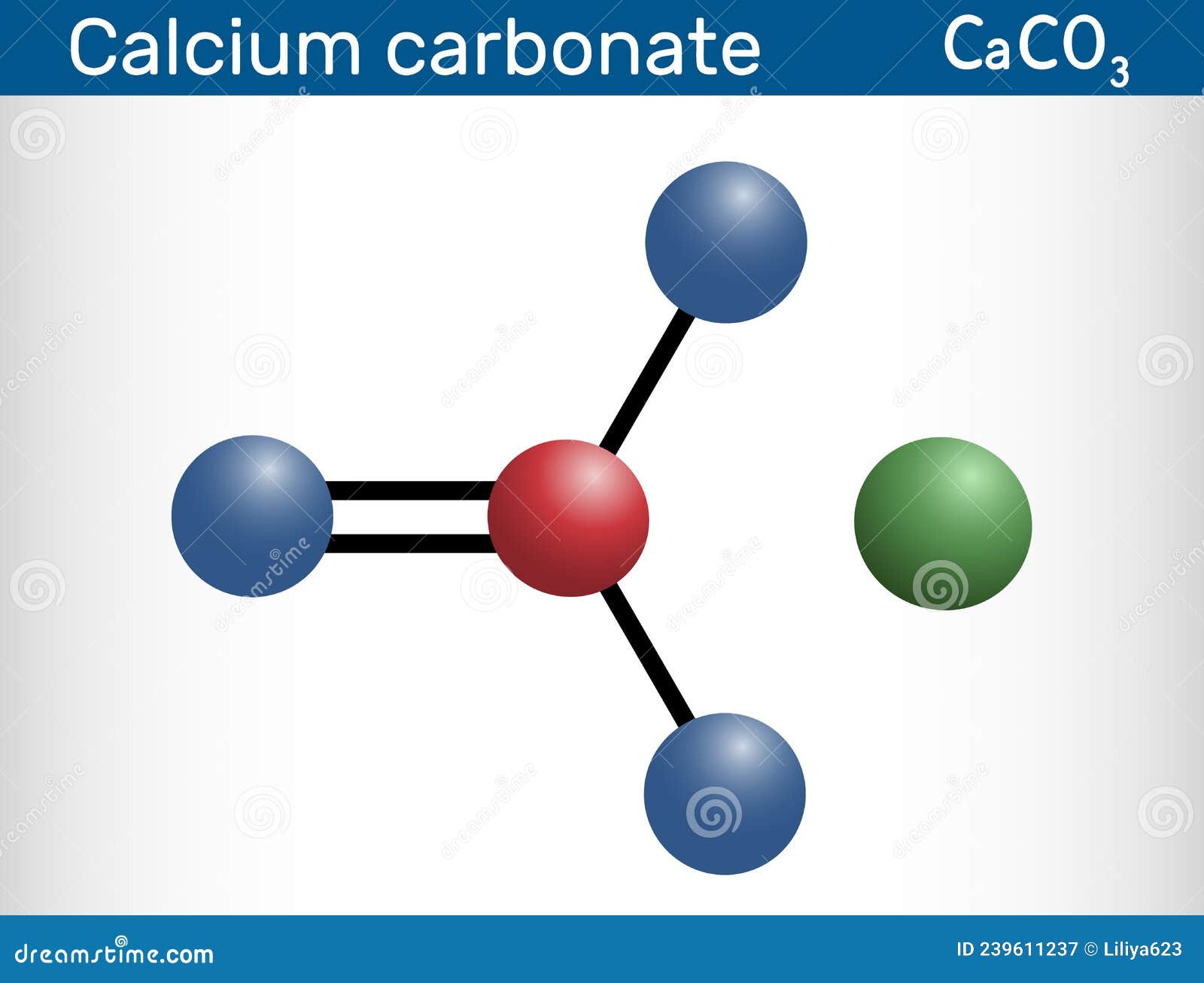
Calcium Carbonate Molecule. it is an Ionic Compound, the Carbonic Salt
Calcium carbonate. Molecular Formula CCaO. Average mass 100.087 Da. Monoisotopic mass 99.947334 Da. ChemSpider ID 9708. - Charge.
Purchase Calcium carbonate [471341] online • Catalog • Molekula Group
To tell if CaCO3 (Calcium carbonate) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Ca is a metal and CO3 is a.

Caco3 calcium carbonate 3D model TurboSquid 1423526
Calcium carbonate is a chemical compound with the formula CaCO3 formed by three main elements: carbon, oxygen, and calcium. It is a common substance found in rocks in all parts of the world (most notably as limestone), and is the main component of shells of marine organisms, snails, coal balls, pearls, and eggshells.
Caco3 molecule fotografías e imágenes de alta resolución Alamy
Appearance Calcium carbonate appears as a white, odorless powder or solid with a crystalline structure. Sample reactions for CaCO3 Related compounds Formula in Hill system is CCaO3 Computing molar mass (molar weight) To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use:
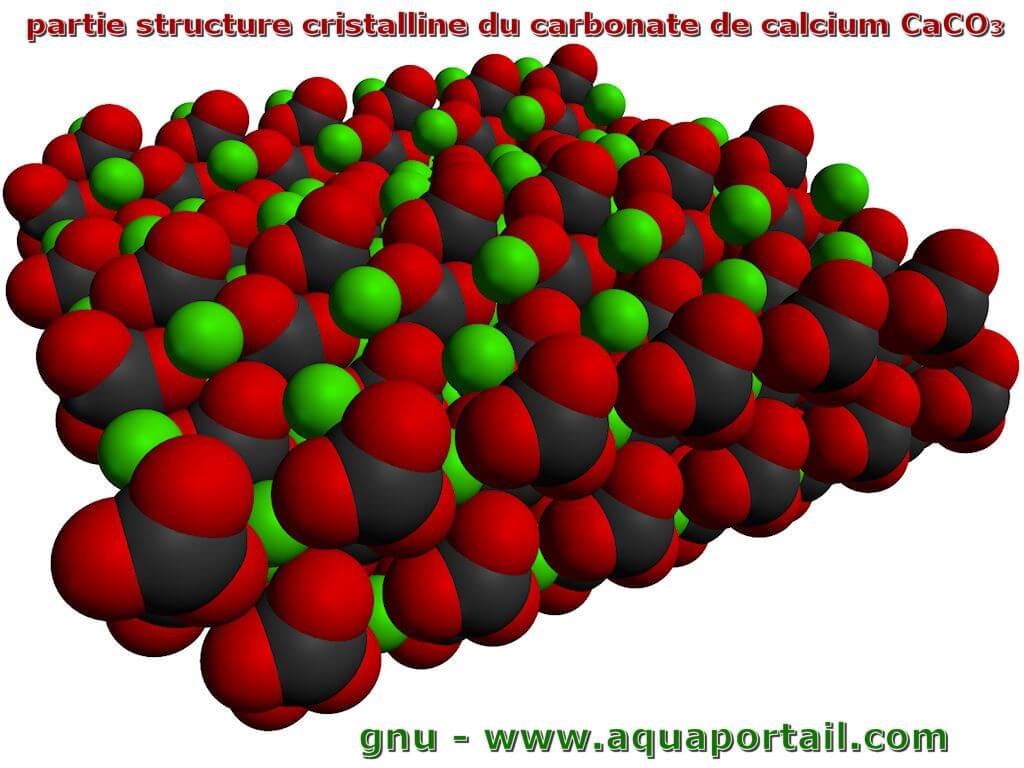
Carbonate de calcium définition et explications
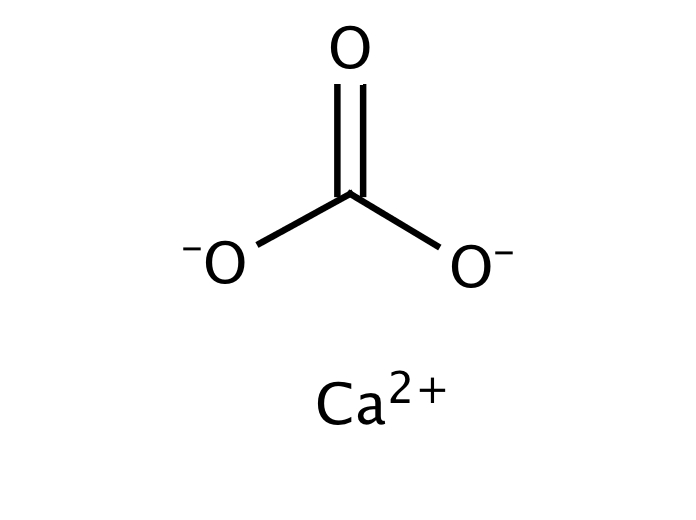
Formula and structure: The calcium carbonate chemical formula is CaCO 3 and its molar mass is 100.0869 g mol-1.The molecule is formed by the calcium cation Ca +2 and the carbonate anion CO 3-2.The structure of the calcium carbonate lattice depends on the mineral from it is extracted: the calcite contains a hexagonal calcium carbonate structure while the aragonite contains orthorhombic lattice.

Calcium carbonate(CaCO3), Honeywell Fisher Scientific
Calcium carbonate Formula: CCaO 3 Molecular weight: 100.087 IUPAC Standard InChI: InChI=1S/CH2O3.Ca/c2-1 (3)4;/h (H2,2,3,4);/q;+2/p-2 IUPAC Standard InChIKey: VTYYLEPIZMXCLO-UHFFFAOYSA-L Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript .

How to Find the Number of Atoms in CaCO3 (Calcium carbonate) YouTube
Precipitated calcium carbonate (CAS: 471-34-1) is produced industrially by the decomposition of limestone to calcium oxide followed by subsequent recarbonization or as a by-product of the Solvay process (which is used to make sodium carbonate).
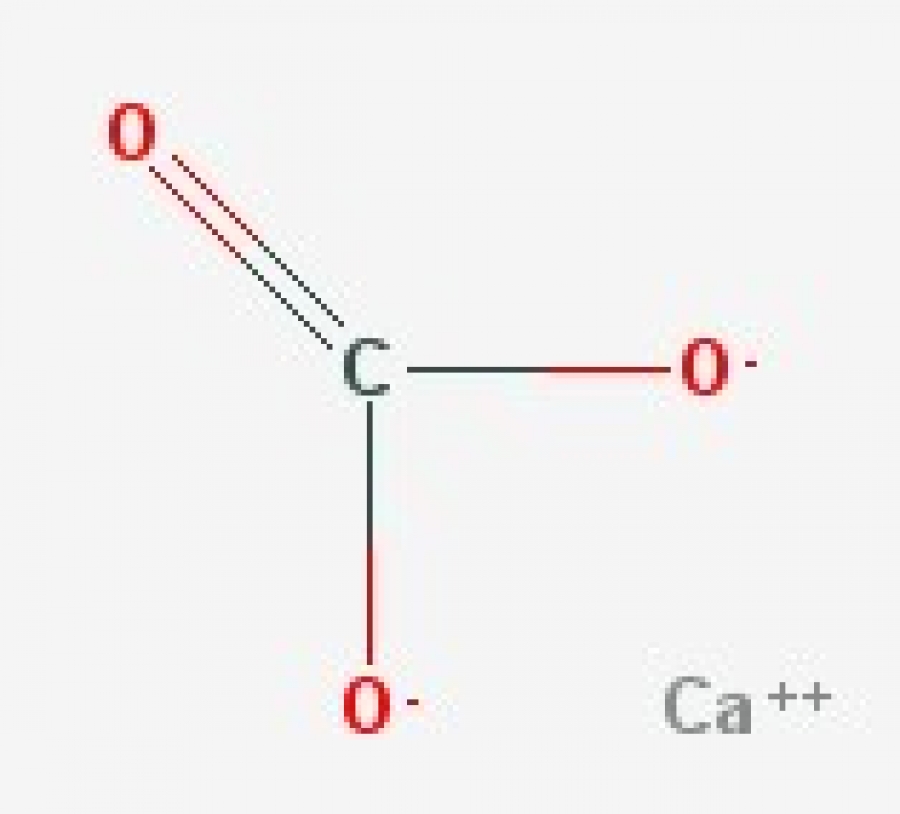
CaCO3 (Calcium Carbonate)
Carbonates. Carbonate is a polyatomic anion with the formula CO2−3 C O 3 2 − and has a trigonal planar molecular structure which consists of a carbon atom surrounded by three oxygen atoms. The carbonate ion is a moderately strong base, so by definition of a Lewis base, it attracts protons in aqueous solutions.

Calcium carbonate molecule. It is ionic compound, carbonic salt of
Calcium carbonate is an inorganic chemical compound with the chemical formula CaCO 3. Calcium carbonate is one of the most popular chemicals which is first encountered in school classrooms, where the use of chalk (a form of CaCO3) is found. It is found in the earth's crust. It is also found in many forms such as marble, limestone, etc.

Calcium Carbonate 3D Model CaCO3 free 3D model CGTrader
Molecular weight calculation: 40.078 + 12.0107 + 15.9994*3 Percent composition by element Element: Calcium Symbol: Ca Atomic Mass: 40.078 # of Atoms: 1 Mass Percent: 40.043% Element: Carbon Symbol: C Atomic Mass: 12.0107 # of Atoms: 1 Mass Percent: 12.000% Element: Oxygen Symbol: O Atomic Mass: 15.9994 # of Atoms: 3 Mass Percent: 47.957%

CaCO3 Chemical & Common Name, Molar & Atomic Mass
Calcium carbonate is an odourless chemical compound. It is a water-insoluble source of calcium. It is mainly found in rocks and is the carbonic salt of calcium. Some of the pure calcium carbonate minerals are Calcite, Vaterite, Aragonite.
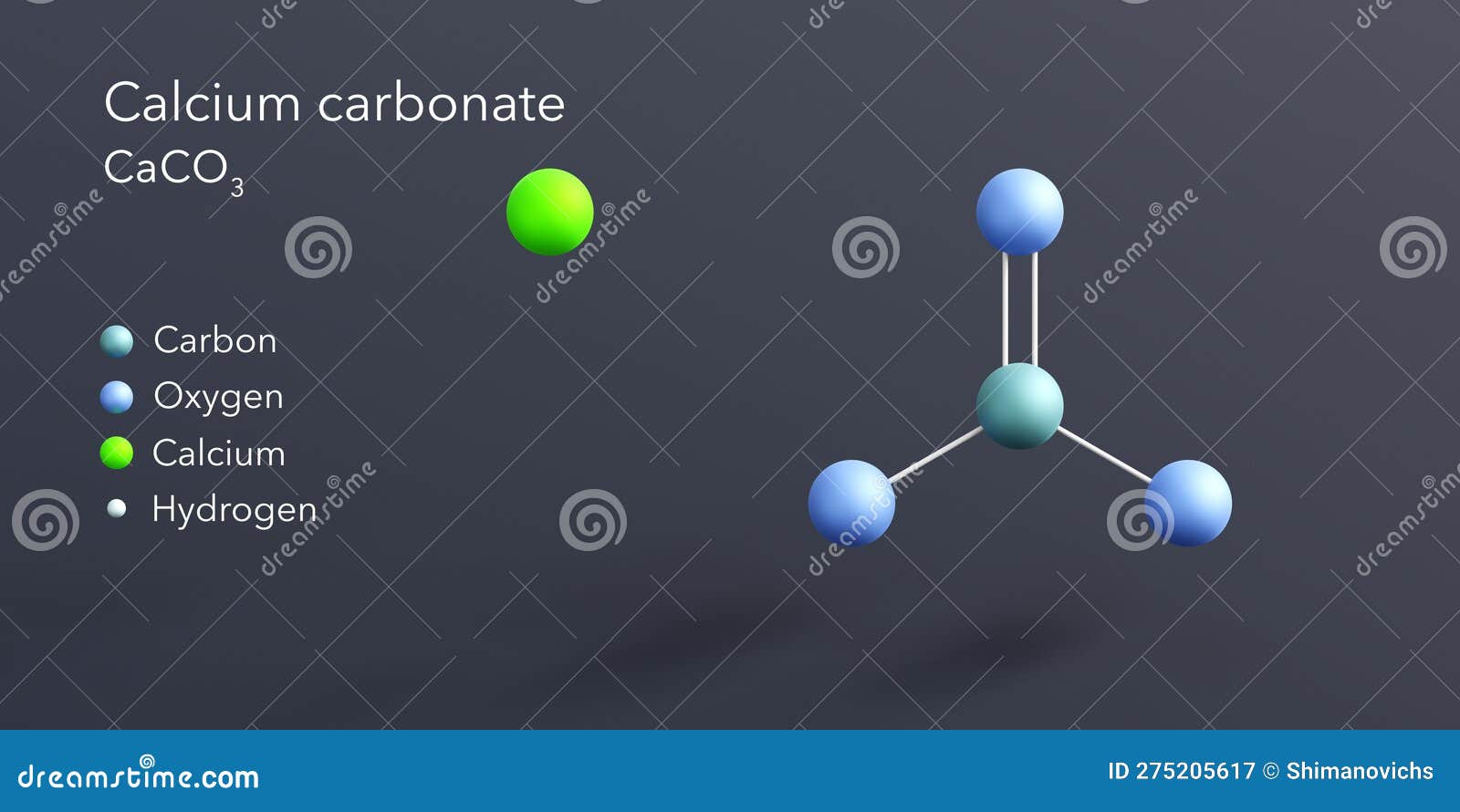
Calcium Carbonate Molecule 3d Rendering, Flat Molecular Structure with
Calcium Carbonate is a chemical compound having the chemical formula CaCO3. It is a white and insoluble powder-like substance that occurs naturally in minerals, marble, chalk, limestone, shells, calcite, pearl, and other related compounds. Medicinally, we use it as an antacid or a calcium supplement. It can also be used as cosmetics fillers.
Fragments of the crystal structures of (a) CaCO3 (calcite), (b
Calcium Carbonate Formula The chemical formula of calcium carbonate is CaCO₃. This means that each molecule of calcium carbonate contains one calcium atom, one carbon atom, and three oxygen atoms. The formula is important in determining the amount of calcium carbonate needed for a specific application and in understanding its chemical properties.

3 CaCO3 Physical Sciences Science
simple solution method 1. Introduction Calcium carbonate (CaCO3) is an abundant inorganic biomaterial in the manner of different structures (calcite, vaterite, and aragonite) [1, 2 ]. Thermodynamically, the form of CaCO 3 under normal conditions is β-CaCO 3 (calcite).
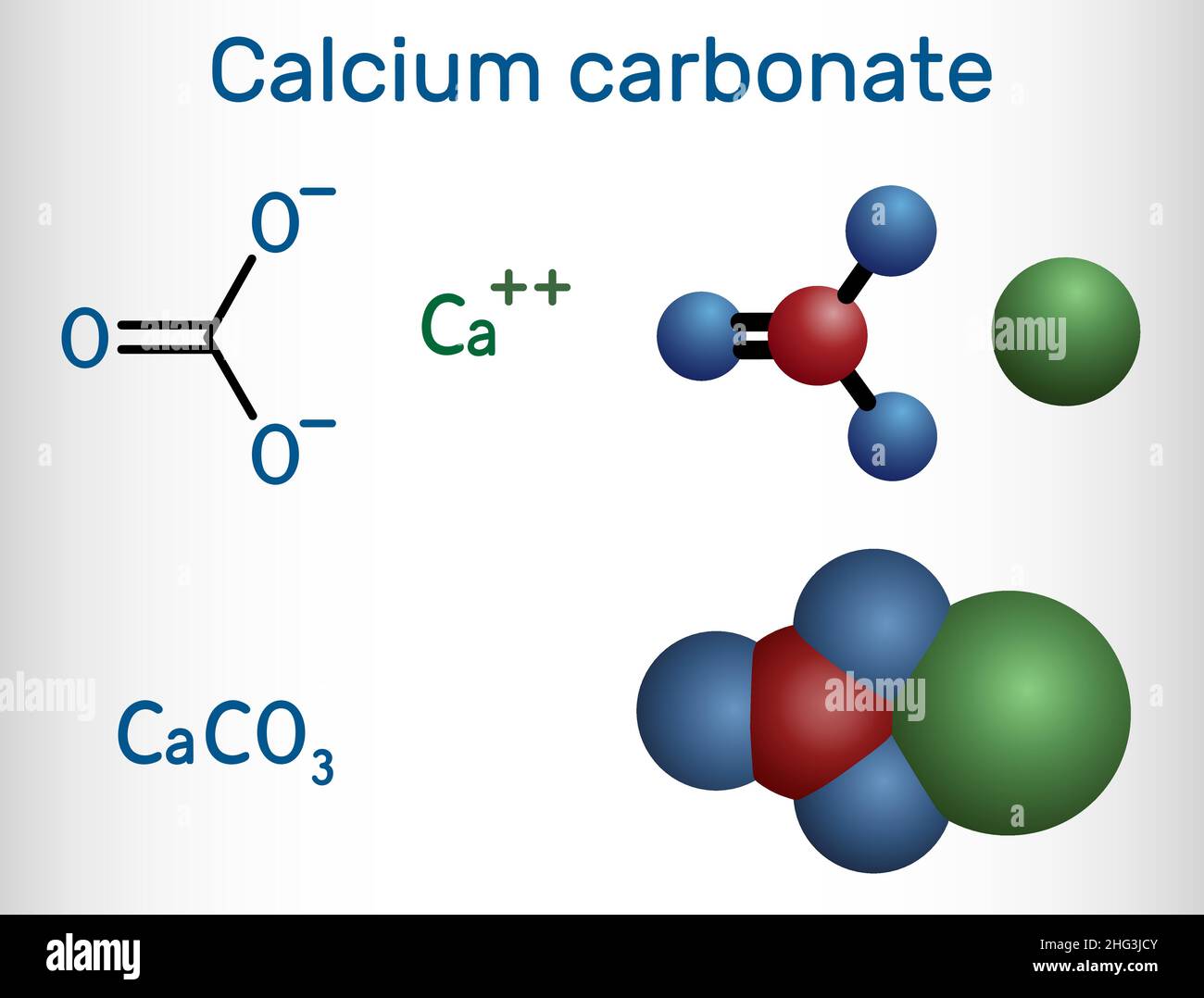
Calcium carbonate molecule. It is an ionic compound, the carbonic salt
Calcium carbonate, CaCO 3, is one of the most common compounds on Earth, making up about 7% of Earth ' s crust. It occurs in a wide variety of mineral forms, including limestone, marble, travertine, and chalk. Calcium carbonate also occurs combined with magnesium as the mineral dolomite, CaMg (CO 3) 2.