Hydrogen atom on white background Royalty Free Vector Image

Difference Between Hydrogen Atom and Hydrogen Ion Compare the Difference Between Similar Terms
Hydrogen. Formula: H 2. Molecular weight: 2.01588. IUPAC Standard InChI: InChI=1S/H2/h1H. Copy Sheet of paper on top of another sheet. IUPAC Standard InChIKey: UFHFLCQGNIYNRP-UHFFFAOYSA-N. Copy Sheet of paper on top of another sheet. CAS Registry Number: 1333-74-. Chemical structure:

Hydrogen atom on white background Royalty Free Vector Image
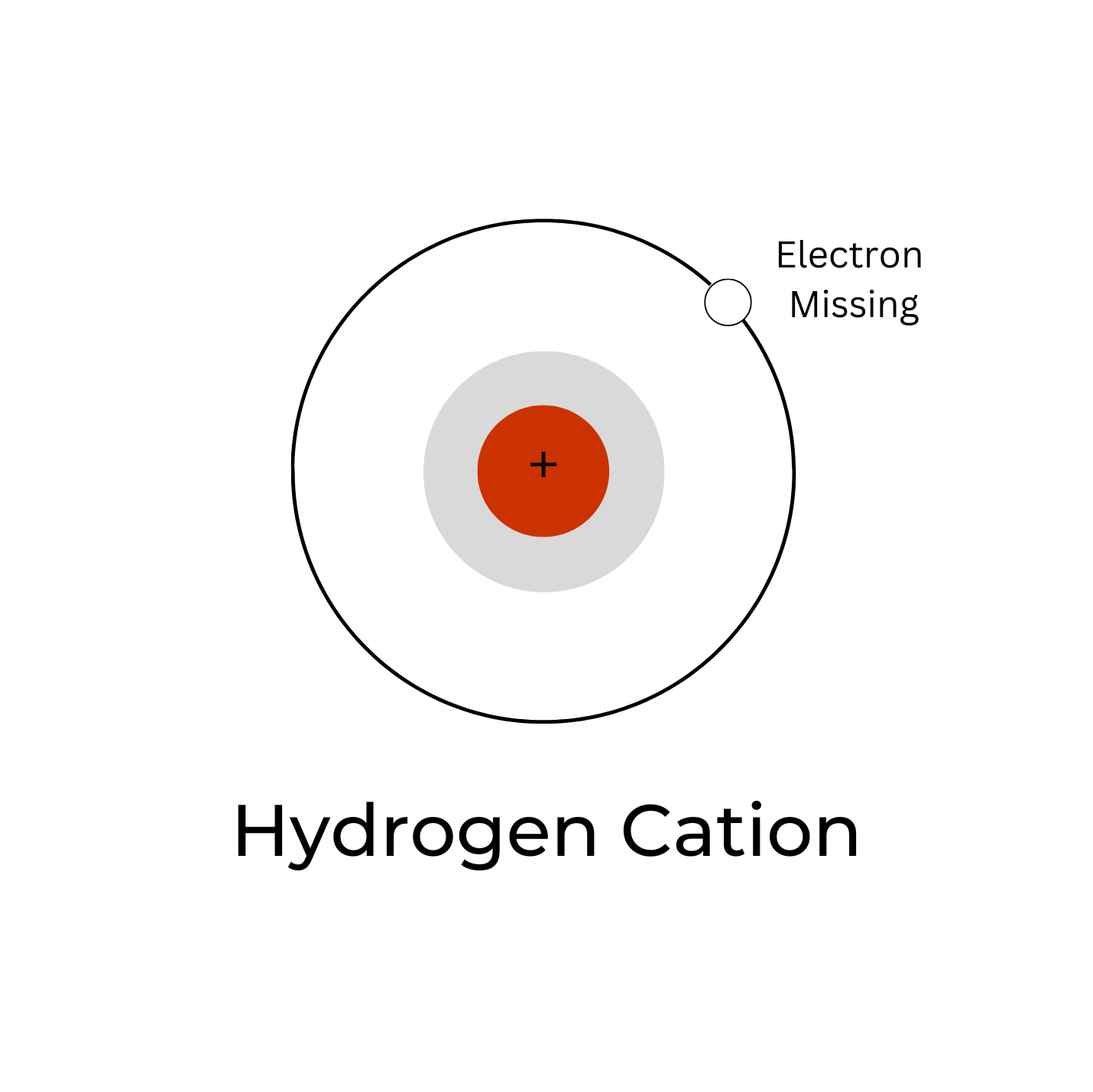
5.2: Hydronium Ions. A hydrogen ion, H +, is a hydrogen atom which has lost its single electron; that is, a hydrogen ion is just a proton. It does not, however, readily exist in an aqueous solution. Because a proton is only about one ten-thousandth as big as an average atom or ion, water dipoles can approach very close to a hydrogen ion in.
Dummies Guide To Hydrogen MHI
The simplest molecule among all is the hydrogen molecular ion H. The ion can be obtained by ionizing H 2 molecules through electron bombardment, for example. In the presence of neutral H 2 molecules, the ion is subjected to a fast chemical reaction, H + 2 + H 2 → H + 3 + H. If this reaction is suspended by some means, the thermodynamically stable H 2 ion can be observed spectroscopically.
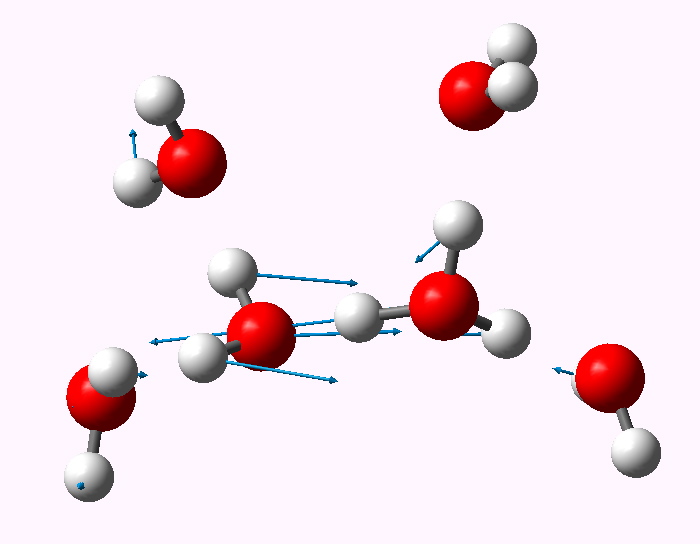
The structure of the hydrogen ion in water. Henry Rzepa's Blog Henry Rzepa's Blog
Ion hidronium. Kation zundel. ketika hidrogen kehilangan elektronnya, kation-kation berikut ini dapat dibentuk: Hidron: nama umum bagi ion positif isotop hidrogen manapun (H +) Proton: 1 H + (lebih tepatnya, kation protium) Deuteron: 2 H +, D +. Triton: 3 H +, T +. Selain itu, ion-ion yang dihasilkan oleh reaksi kation-kation ini dengan air.

How to Draw the Lewis Dot Structure for H+ (Hydrogen ion) YouTube
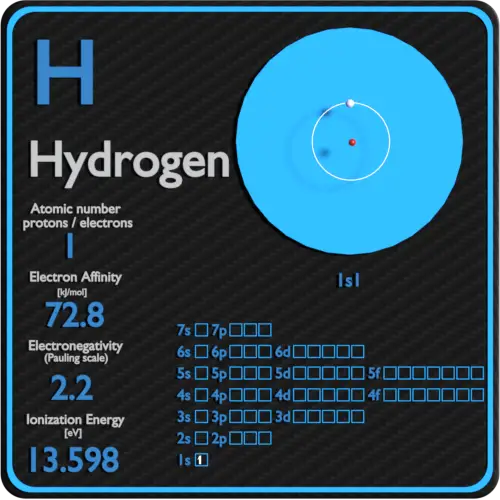
Atomic Number: 1. Hydrogen is the first element in the periodic table, meaning it has an atomic number of 1 or 1 proton in each hydrogen atom. The name of the element comes from the Greek words hydro for "water" and genes for "forming," since hydrogen bonds with oxygen to form water (H 2 O). Robert Boyle produced hydrogen gas in 1671 during an.
Hydrogen Periodic Table and Atomic Properties
There is a direct relationship between hydrogen ions and pH. The pH of a solution depends on the hydrogen ion concentration in that solution. pH value is the logarithmic value of the inverse of the hydrogen ion activity. Since the concentration of the hydrogen ions is often very low, ion activity is considered as equal to the concentration of.

What’s So Great About Molecular Hydrogen? by KOR Water Medium
Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest element and, at standard conditions, is a gas of diatomic molecules with the formula H2, sometimes called dihydrogen, [10] but more commonly called hydrogen gas, molecular hydrogen or simply hydrogen. It is colorless, odorless, tasteless, [11] non-toxic, and.

Ion hidrógeno
A hydrogen ion is created when a hydrogen atom loses an electron.A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle-free space. Due to its extremely high charge density of approximately 2×10 10 times that of a sodium ion, the bare hydrogen ion cannot exist freely in.

Diagram representation element hydrogen Royalty Free Vector
The dihydrogen cation or hydrogen molecular ion is a cation (positive ion) with formula H+. 2. It consists of two hydrogen nuclei ( protons) sharing a single electron. It is the simplest molecular ion . The ion can be formed from the ionization of a neutral hydrogen molecule ( H. 2) by electron impact. It is commonly formed in molecular clouds.
Hydrogen atom orbital structure hires stock photography and images Alamy
It has the lowest density of all gases. Uses. Some see hydrogen gas as the clean fuel of the future - generated from water and returning to water when it is oxidised. Hydrogen-powered fuel cells are increasingly being seen as 'pollution-free' sources of energy and are now being used in some buses and cars.

Hydrogen Bond Definition, Types, and Examples
Depiction of a hydrogen atom showing the diameter as about twice the Bohr model radius. (Image not to scale) A hydrogen atom is an atom of the chemical element hydrogen.The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the.

Diagram Representation of the Element Hydrogen Stock Vector Illustration of
A hydrogen atom that has gained or lost an electron, and thus has an electric charge. The positive hydrogen ion, H +, has lost its only electron and therefore consists of a single proton. A nebula containing H + ions is known as an H II region. The negative hydrogen ion, H −, has gained a second electron. H − ions are found in the outer.

Hydrogen ion YouTube
The glass electrode for H + is an ion-selective electrode (ISE) widely used in clinical medicine. The potential that develops in this electrode is proportional to the log of the hydrogen ion activity in the test solution. The term used is pH which is now defined as: Definition: pH. pH = −log10 aH+ (1.3.2) (1.3.2) p H = − log 10 a H +.
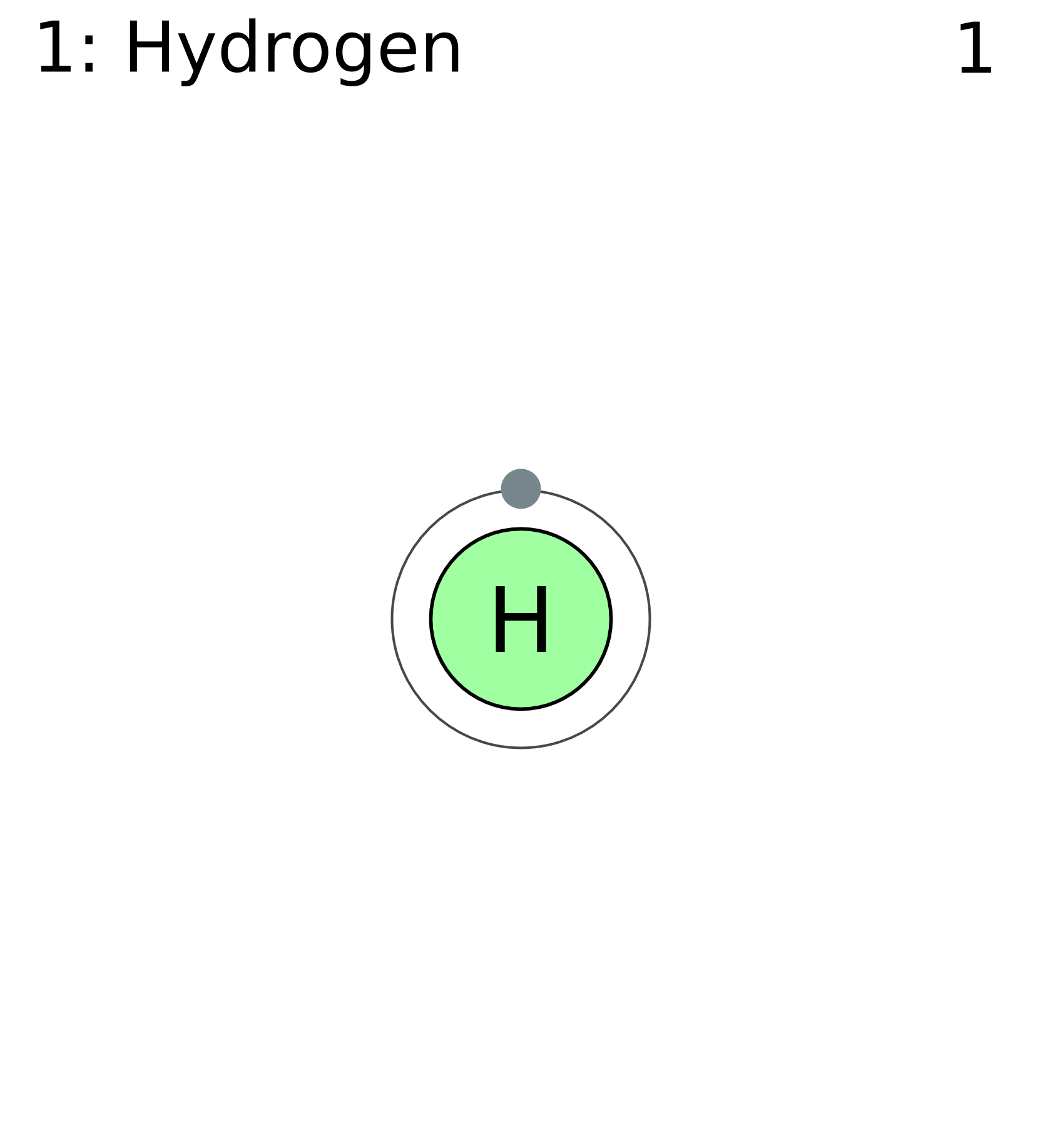
FileElectron shell 001 hydrogen.png
hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron.The hydrogen nucleus is made up of a particle carrying a unit positive electric charge, called a proton.The isolated hydrogen ion, represented by the symbol H +, is therefore customarily used to represent a proton.Because the bare nucleus can readily combine with other particles (electrons, atoms.
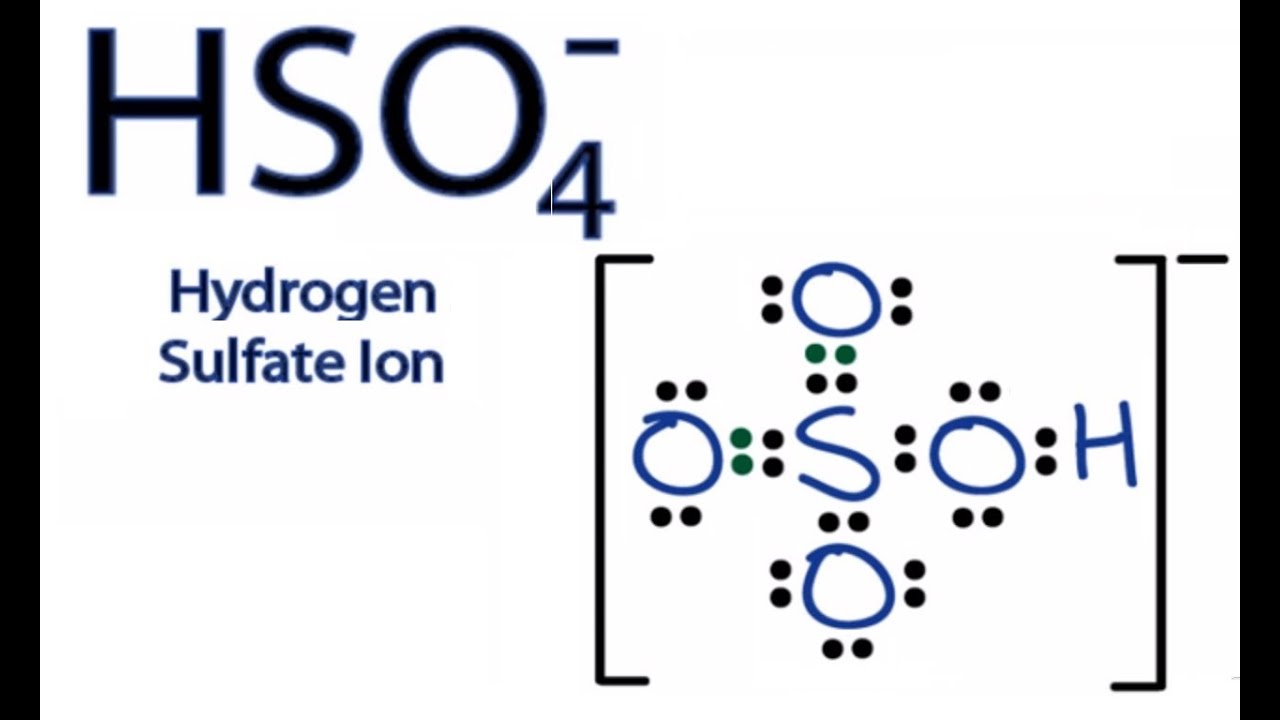
Hydrogen Hydrogen Ion Formula
13.3: Hydrogen Molecule Ion. Page ID. Richard Fitzpatrick. University of Texas at Austin. The hydrogen molecule ion consists of an electron orbiting about two protons, and is the simplest imaginable molecule. Let us investigate whether or not this molecule possesses a bound state: that is, whether or not it possesses a ground-state whose energy.

Hydrogen Definition, Structure, Properties & Uses Embibe
A hydrogen ion is the nucleus of a hydrogen atom that has been isolated from its electron. A proton is a molecule with a unit of positive electric energy that makes the hydrogen nucleus. Therefore, the disconnected hydrogen ion, signified by the image H +, is usually used to depict a proton.So the above-given definition assists with getting what is H + ion and hydrogen ion Formula.